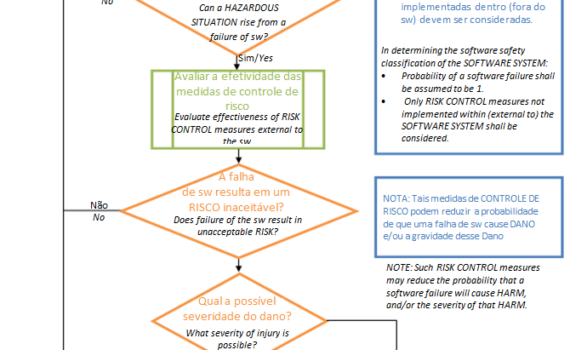
Hoje iremos ver como a IEC 62304 trata o processo de desenvolvimento de software desde o planejamento até o lançamento do dispositivo médico. Alguns requisitos dependem da classificação de segurança do software ( trato disto no primeiro post desta série "Processo de ciclo de vida de software de equipamento Médico […]