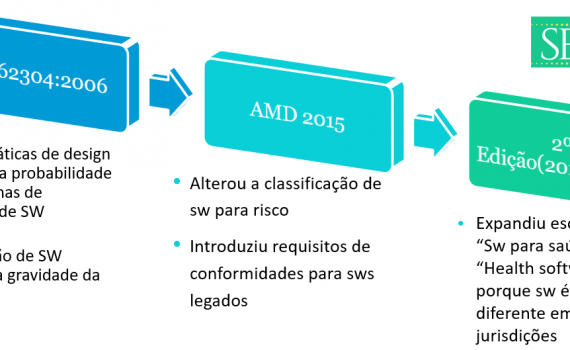
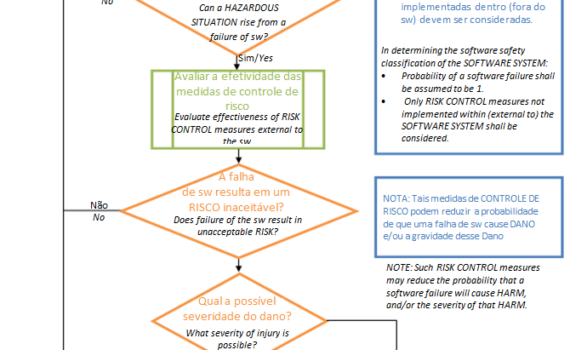
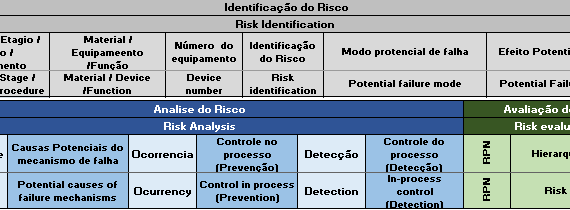
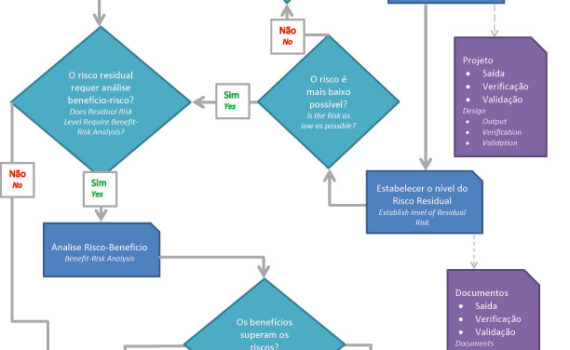
Remember to update the references of the Anvisa RDCs that came into effect this October. Fortunately, there was no change in content, there was only an alignment to consolidate other norms on specific matters. New Affects Effective in IN 101/2021 Revokes IN 6/2011 and IN 13/2016 […]