
- => Define your risk management process
- => Establish management rules and responsibilities
- => Document the risk management plan
- => Establish a live risk management file

- => Understand and define the scope of your device and document the intended use

- => Identify the potential sources of damage associated with your product. This is known as danger

- => Estimate the risk of each dangerous situation.

- => Risk is the combination of the severity of the potential damage and the probability of the damage occurring

- => Are the risks at an acceptable level?
- => Is risk reduction necessary?
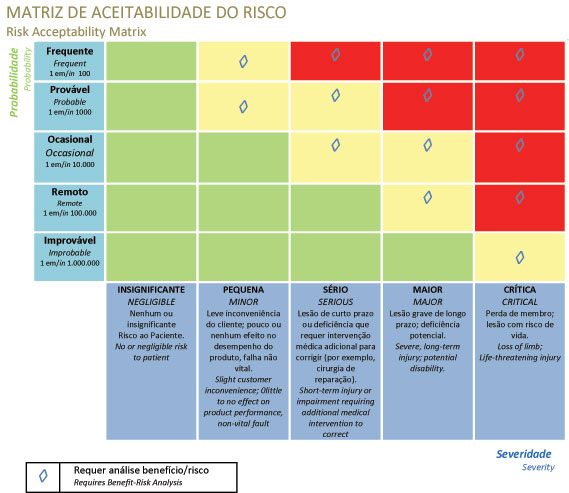

- => Use risk control to reduce risk to acceptable levels.
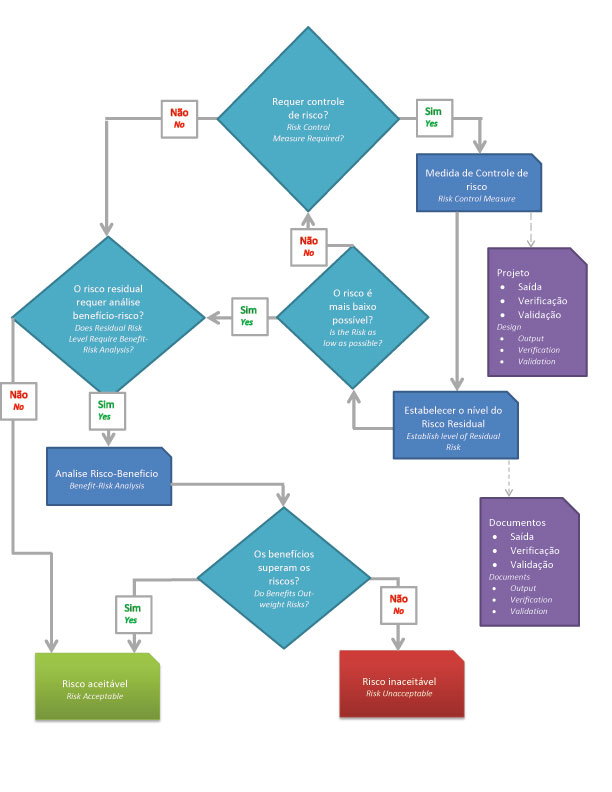

- => Assess the risk of the product in its entirety.
- => Is the risk level acceptable?
- => Does the benefit outweigh the potential risks?

- => Perform a risk management analysis and prepare a risk management report before sending your device to commercial production.

- => Internal auditing, Preventive and corrective actions, compliance, customer feedback, material non-compliance, all sources in the risk management process.
- => Risk management is a process for the entire product life cycle.
In the next publication, I will write about practical tips for a good risk analysis meeting.Leave your comment and until the next post.





