Part 1- Risk management is for the product cycle process of the product
Risk and medical equipment
The patient accepts the risks of the medical device you and I project, develop and manufacture.
And that's exactly why risk management is so important to the medical device industry.
Risk management allows you to know that the medical devices you are involved in bringing patients and end users are safe and can be used as a tool to help us design, develop and manufacture safer medical devices.
Medical device regulatory agencies have placed the topic management in the foreground and most of them uses ISO 14971 for this.
ISO 14971 - Health Products - Application of Risk Management to Health Products
The purpose of this standard is to help medical device manufacturers set a risk management process they can use to:
- >Identificar perigos
- >Estimar e avaliar riscos
- >Desenvolver, implementar e monitorar a eficácia da medida de controle de risco
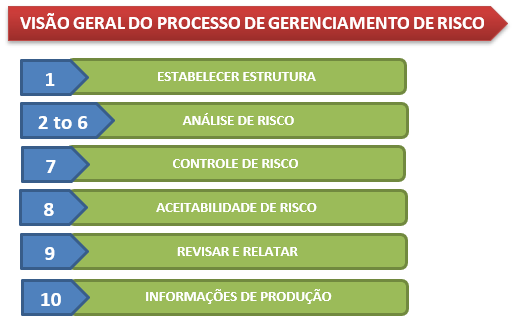
In the next publication, I will describe each step of this process. Leave your comment and until the next post.


